More Than Access
More Than Access (MTA) S.r.l. Società Benefit is a boutique consulting firm specialized in providing services that facilitate market access for healthcare technologies. MTA is founded upon the idea of building healthy partnerships with scientific societies, Institutions and academia, patient associations and companies offering complementary services and is oriented to the European context through the development of strategic collaborations with agencies in other countries
Based on the knowledge of the target market and the analysis of its continuous evolution, MTA intends to offer customized solutions to support all stakeholders in the drug supply chain and in particular pharmaceutical companies-whether Big Pharma or start-ups-in order to optimize the access of health technologies up to the patient, in an effective, timely and sustainable way.
events

HEALTHforFUTURE
Shaping Healthcare from Education to Business
DATE: June 17-18, 2024
CONFERENCE VENUE: Hotel NH Bergamo
SCIENTIFIC COORDINATOR:
Paola Minghetti, Full Professor of Pharmaceutical Technology and Legislation, Director of the Department of Pharmaceutical Sciences, Faculty of Pharmaceutical Sciences, University of Milan - Sitelf President - AFI Vice-President

PHARMATREND 2025
Early Access Progams: it’s time for a new deal
DATE: 12-13 Marzo 2025
CONFERENCE VENUE: Park Hotel Villa Ferrata (Grottaferrata – RM)
SCIENTIFIC COORDINATOR:
Armando Genazzani, Professore di Farmacologia, Dipartimento di Scienze e Tecnologia del Farmaco, Università degli Studi di Torino
Evento organizzato in collaborazione con UNITO

COMBOCONNECT
Bridging Access Gaps in Combination Therapies
DATE: 26 Marzo 2025
CONFERENCE VENUE: Istituto Luigi Sturzo – Palazzo Baldassini, Via delle Coppelle 35, Roma
RESPONSABILI SCIENTIFICI:
Proff. Pier Luigi Canonico e Claudio Jommi
Evento di presentazione del Report, realizzato in collaborazione con ISPOR Italy-Rome Chapter
Our firm is based on solid values
oriented toward excellence in consulting.
Boutique consulting firm
We are proud to be a boutique consulting firm, as it allows us to offer a highly personalized and dedicated service to our clients. This enables us to fully understand their specific needs and provide tailored, quick and creative solutions that generate added value.
Client orientation
We always make the client the focus of everything we do. We listen carefully to requests, needs and ambitions, and analyze everything critically, to develop strategies and action plans until we deliver the best possible solution, always creating partnerships based on mutual trust.
Community value
we are constantly striving to improve, including through research and/or nonprofit activities, the health status of patients and the sustainability of the National Health Service, by promoting, supporting and enabling initiatives intended to create value in the community and have a positive impact in terms of social value..
Business ethics
We are proud to be a Benefit Corporation, which emphasizes our commitment to maintaining a positive impact on the community. Our consulting is based on ethical principles and a responsible approach, which allows us to operate with integrity, transparency and respect for all parties involved.
MTA Code of Ethics
Model of Organization, Management and Control pursuant to D.Lgs. 231/2001
We are passionate in what we do and constantly strive to exceed our clients' expectations by providing high-level consulting services that help them succeed in the complex healthcare landscape.
We are focused and oriented on creating the best possible conditions for inclusiveness,
flexibility and quality of work, to attract, grow and retain talents, enhancing their skills and aptitudes, not only exquisitely job-related ones, favoring the use of innovative and technological solutions aimed at implementing a work-life integration approach.
meet the team
Dario Lidonnici | Founder & Managing Director
Giorgio Casilli | Co-Founder & Market Access Lead
Giorgio is an experienced Market Access professional with a solid academic background (Master's Degree in Pharmacy, 2nd Level Master's Degree in Regulatory Disciplines and Market Access in Pharmaceuticals). Over the years, Giorgio has rapidly progressed in his career, providing strategic advice and detailed analysis for numerous projects in multiple therapeutic areas. His expertise has also been established through a strong research interest by authoring scientific articles and participating in conferences, proving his commitment to in-depth, continuous professional development and knowledge sharing in the field. The combination of his academic education, professional experience, and commitment to scientific research make Giorgio a passionate and qualified professional, ready to bring a fresh and innovative perspective.
Armando Isernia | Regional Market Access Director
Decades of experience in the pharmaceutical world, with increasing responsibilities in the areas of sales and access, in a multinational company. After several years as Head of Regional Market Access at AstraZeneca, since July 2012 he has taken on the role of Regional Market Access Director & Partner at MA Provider, dealing with both access consultancy and staff training for pharmaceutical and device companies. During his career, Armando has also been a lecturer in various training courses in different masters.
Roberto Ravasio | HEOR and RWE Lead
Roberto has more than two decades of experience in the field of Market Access, Health Economics and Outcomes Research, Pharmacoeconomics, Health Technology Assessment. Roberto developed specific skills in cost-effectiveness analysis, budget impact analysis, drug utilization analysis, real world data analysis, Delphi Panel, strategic consultancy and training. Roberto has also been a lecturer in various training courses in different masters. Roberto is the co-Editor of Global & Regional Health Technology Assessment and the Editor of AboutOpen magazine HTA section. He is currently HEOR & RWE Lead at MTA.
Marika De Nigris | Market Access Manager
Marika has a master’s degree in Healthcare Management. After initial work experiences at Sanofi and Boston Scientific, Marika decided to develop her attitude towards consultancy, joining a first leading healthcare consulting firm and then MTA with the role of Market Access Manager. After four years of experience, Marika is able to deal with complex projects, directly interacting with KOLs, payers and clients, and managing the whole execution of P&R dossiers in different therapeutic areas.
Andrea Torriani | Market Access Manager
Andrea holds a master’s degree in Pharmaceutical Chemistry and Technology and demonstrated an early passion for market access consultancy. He joined a leading healthcare consulting firm during his academic path, first performing his master thesis project and then starting his professional career. With over three years of substantial experience in the field, Andrea has assumed the role of Market Access Manager at MTA. Here, he continues the play an integral part in numerous successful projects spanning several therapeutic areas.
Alessia Dinoia | Market Access Specialist
Alessia holds a degree in Pharmaceutical Chemistry and Technology and during her studies had a brief experience as a pharmacist and oncology researcher. She later earned a 2nd Level Master’s Degree in Healthcare Management, Pharma and Biomed.
After a two years experience in a consulting firm in Life Science and Healthcare, now with passion and enthusiasm, she joins such as Market Access Specialist.
Edoardo Sanna | Market Access Specialist
Edoardo holds a degree in Pharmacy and a 2nd Level Master’s Degree in Market Access and Regulatory Disciplines. After gaining experience as a pharmacist and consultant in the Lifescience sector, he now serves as a Market Access Specialist at MTA.
His skill in finding innovative solutions to complex situations, along with his curiosity and drive for continuous improvement, motivates him to contribute with dedication to the success of the projects he works on.
Muriel Bertomoro | Business Support Manager
Muriel has more than 15 years experience in the administrative and organizing fields.
After an initial 3-years experience in the commercial-marketing field, Muriel worked for 12 years for the Italian Society of Pharmacology and the Italian Society of Toxicology, both non-profit associations in the medical-scientific field, covering the roles of Administrative, Associates and Fundraising Manager.
She currently holds the role of Business Support Manager at MTA.
Tommaso Cao | Market Access Junior Specialist
Tommaso holds a master’s degree in Pharmaceutical Chemistry and Technology from the University of Piemonte Orientale. After graduating, he began his career at the Clinical Trial Center of the Hospital “Maggiore della Carità” in Novara, where he was responsible for contract negotiations and documentation management for clinical trials.
In April 2024, Tommaso joined MTA to expand his knowledge in the market access field, while also pursuing a second-level Master's in Regulatory Affairs and Market Access, further strengthening his expertise.
Aureliano Stingi | Senior Project Manager
Aureliano is a cancer biologist and science communication expert with a diverse background in oncology, ranging from basic research to clinical applications.
Aureliano contributing regularly as a scientific journalist for Repubblica, where he covers topics such as COVID-19, aging, and health. He has hosted several health-focused podcasts in collaboration with leading pharmaceutical companies.
With a PhD in Cancer Biology and an MBA, Aureliano combines his scientific expertise with a deep understanding of the medical market, making him informed in both the research and business aspects of healthcare.
meet the team
Dario Lidonnici | Founder & Managing Director
Giorgio Casilli | Co-Founder & Market Access Lead
Giorgio is an experienced Market Access professional with a solid academic background (Master's Degree in Pharmacy, 2nd Level Master's Degree in Regulatory Disciplines and Market Access in Pharmaceuticals). Over the years, Giorgio has rapidly progressed in his career, providing strategic advice and detailed analysis for numerous projects in multiple therapeutic areas. His expertise has also been established through a strong research interest by authoring scientific articles and participating in conferences, proving his commitment to in-depth, continuous professional development and knowledge sharing in the field. The combination of his academic education, professional experience, and commitment to scientific research make Giorgio a passionate and qualified professional, ready to bring a fresh and innovative perspective.
Marika De Nigris | Market Access Manager
Marika has a master’s degree in Healthcare Management. After initial work experiences at Sanofi and Boston Scientific, Marika decided to develop her attitude towards consultancy, joining first Pharmalex (formerly MA Provider) and then MTA with the role of Market Access Manager. After four years of experience, Marika is able to deal with complex projects, directly interacting with KOLs, payers and clients, and managing the whole execution of P&R dossiers in different therapeutic areas.
Andrea Torriani | Market Access Manager
Andrea holds a master’s degree in Pharmaceutical Chemistry and Technology and demonstrated an early passion for market access consultancy. He joined Pharmalex (formerly MA Provider) during his academic path, first performing his master thesis project and then starting his professional career. With over three years of substantial experience in the field, Andrea has assumed the role of Market Access Manager at MTA. Here, he continues the play an integral part in numerous successful projects spanning several therapeutic areas.
Armando Isernia | Regional Market Access Director
Decades of experience in the pharmaceutical world, with increasing responsibilities in the areas of sales and access, in a multinational company. After several years as Head of Regional Market Access at AstraZeneca, since July 2012 he has taken on the role of Regional Market Access Director & Partner at MA Provider, dealing with both access consultancy and staff training for pharmaceutical and device companies. During his career, Armando has also been a lecturer in various training courses in different masters.
Roberto Ravasio | HEOR and RWE Lead
Roberto has more than two decades of experience in the field of Market Access, Health Economics and Outcomes Research, Pharmacoeconomics, Health Technology Assessment. Roberto developed specific skills in cost-effectiveness analysis, budget impact analysis, drug utilization analysis, real world data analysis, Delphi Panel, strategic consultancy and training. Roberto has also been a lecturer in various training courses in different masters. Roberto is the co-Editor of Global & Regional Health Technology Assessment and the Editor of AboutOpen magazine HTA section. He is currently HEOR & RWE Lead at MTA.
Alessia Dinoia | Market Access Specialist
Alessia holds a degree in Pharmaceutical Chemistry and Technology and during her studies had a brief experience as a pharmacist and oncology researcher. She later earned a 2nd Level Master’s Degree in Healthcare Management, Pharma and Biomed.
After a two years experience in a consulting firm in Life Science and Healthcare, now with passion and enthusiasm, she joins such as Market Access Specialist.
Edoardo Sanna | Market Access Specialist
Edoardo holds a degree in Pharmacy and a 2nd Level Master’s Degree in Market Access and Regulatory Disciplines. After gaining experience as a pharmacist and consultant in the Lifescience sector, he now serves as a Market Access Specialist at MTA.
His skill in finding innovative solutions to complex situations, along with his curiosity and drive for continuous improvement, motivates him to contribute with dedication to the success of the projects he works on.
Muriel Bertomoro | Business Support Manager
Muriel has more than 15 years experience in the administrative and organizing fields.
After an initial 3-years experience in the commercial-marketing field, Muriel worked for 12 years for the Italian Society of Pharmacology and the Italian Society of Toxicology, both non-profit associations in the medical-scientific field, covering the roles of Administrative, Associates and Fundraising Manager.
She currently holds the role of Business Support Manager at MTA.
Tommaso Cao | Market Access Junior Specialist
Tommaso holds a master’s degree in Pharmaceutical Chemistry and Technology from the University of Piemonte Orientale. After graduating, he began his career at the Clinical Trial Center of the Hospital “Maggiore della Carità” in Novara, where he was responsible for contract negotiations and documentation management for clinical trials.
In April 2024, Tommaso joined MTA to expand his knowledge in the market access field, while also pursuing a second-level Master's in Regulatory Affairs and Market Access, further strengthening his expertise.
Aureliano Stingi | Senior Project Manager
Aureliano is a cancer biologist and science communication expert with a diverse background in oncology, ranging from basic research to clinical applications.
Aureliano contributing regularly as a scientific journalist for Repubblica, where he covers topics such as COVID-19, aging, and health. He has hosted several health-focused podcasts in collaboration with leading pharmaceutical companies.
With a PhD in Cancer Biology and an MBA, Aureliano combines his scientific expertise with a deep understanding of the medical market, making him informed in both the research and business aspects of healthcare.

01 Market Access Plan
We develop targeted strategies for healthcare technology market access, with due consideration for the dynamics of the specific field and especially the patients' needs.
02 Negotiation strategy
We provide support in establishing effective negotiation strategies to achieve suitable pricing & reimbursement in line with client expectation.
03 Stakeholder mapping
We identify and engage key players in the field, such as regulatory authorities, payers, and patient associations, in order to create strategic partnerships and maximize market access opportunities.
01 P&R dossier drafting
With expertise in drafting several P&R dossiers in multiple therapeutic areas, we are among the top experts in Pricing & Reimbursement dossier drafting ensuring an accurate and persuasive presentation of the product's value proposition to obtain approval and reimbursement at the best conditions.
02 Regional dossier drafting
We support the creation of region-specific dossiers, considering local peculiarities and the needs of the relevant authorities.
03 Advisory board management
We organize and manage national and regional advisory boards in order to gain valuable insights and obtain qualified feedback from engaged experts.
04 Stakeholder engagement
We actively manage relationships with industry KOLs, facilitating dialogue and collaboration to achieve shared results.
Training
We provide customized training programs for companies and healthcare professionals to deepen their knowledge in the areas of Market Access, Pricing & Reimbursement, and Health Technology Assessment (HTA).
Events
We organize events focused on market access and related topics, such as conferences, workshops and webinars, which provide opportunities to learn from the best minds in the industry, share experiences and networking.
research projects
At MTA, we believe we can contribute to the advancement of knowledge, and therefore we collaborate with experts in the field and academic institutions to conduct original studies while actively contributing to the growth of the scientific community and the promotion of independent research.
In this section you will find a collection of our research work, including publications, reports, abstracts, and posters presented at national and international Congresses.

01Publication
Casilli G, Lidonnici D, Jommi C, De Nigris M, Genazzani AA. Do France, Germany, and Italy agree on the added therapeutic value of medicines? International Journal of Technology Assessment in Health Care. 2023;39(1):e54. doi:10.1017/S026646232300048X

Report
COMBOCONNECT – Bridging Access Gaps in Combination Therapies, Marzo 2025

02Report
EARLY ACCESS PROGRAM: BETWEEN CRITICS AND PROPOSALS FOR THE FUTURE - Report for ISPOR Italy Rome Chapter, DECEMBER 2023
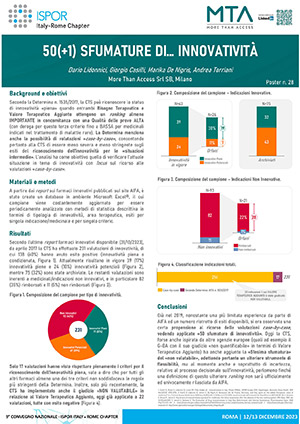
03Poster
Lidonnici D, Casilli G, De Nigris M, Torriani A, 50(+1) SHADES OF... INNOVATIVITY, Presented at National Conference ISPOR ITALY - ROME CHAPTER 12-13 DECEMBER 2023
MTA as a boutique consulting firm stands out for direct accessibility and building strong partnerships with clients. Our distinctive feature is a streamlined approach and direct involvement in each project, providing extensive experience and recognized expertise to ensure customized, high-level consulting services. We do not simply provide standardized solutions but create tailored strategies that reflect our clients' business realities. We are dedicated to supporting our clients not only for specific projects, but for long-term success in the Market Access field.
With an eye on creating value for the community, the core values behind building strong partnerships with clients are speed, creativity, in-depth knowledge of the players and the evolving environment, transparency, accountability, commitment to results and focus on customers and their needs, professionalism, and compliance.
Our experience in market access for a wide range of healthcare technologies, including innovative medicines and advanced therapies, has enabled us to acquire expertise on a number of issues and therapeutic areas, each time characterized by unique challenges that require a focused approach. We have worked with many companies, both big biopharmaceutical companies and start-ups, demonstrating our ability to adapt to different contexts and offer customized solutions for our clients. With our strong network of strategic partnerships with leading experts in the field, we are able to offer a competitive advantage and unique perspective in market access projects.
Do you have any questions? Write to us. Do you have any questions? Write to us. Do you have any questions? Write to us. Do you have any questions? Write to us. Do you have any questions? Write to us.
CONTACTS
info@morethanaccess.com
Registered office: Via Cesare Manigli, 2 - 20121 Milan
Operational headquarter: Via G. Carducci, 24 – 20123 Milan


